
As COVID-19 cases in India cross 4-lakh mark, Modi encourages yoga for improving immunity on ‘International Day of Yoga’
As per the latest updates shared by the Ministry of Health & Family Welfare (MoHFW) today, i.e., Sunday, June 21, 2020, India has crossed the 4-lakh mark of Coronavirus (COVID-19) cases.
The total number of cases in India are now 4,10,461 and there have been 13,254 casualties. In the past 24 hours, India again recorded the highest single day spike with 15,413 cases and 306 casualties.
The recovery rate of the outbreak has also improved to 55.49%. A total of 2,27,755 patients have been cured. During the last 24 hours, a total of 13,925 people were cured. The number of samples being tested everyday has also increased. In the last 24 hours, 1,90,730 samples have been tested. The total number of samples tested is 68,07,226.

Meanwhile, Maharashtra, the worst-hit State by the pandemic, alone reported 3,874 fresh cases, its highest ever jump in a single day and 91 casualties. Of this, Pune alone reported 823 fresh cases and 24 casualties. The latest spike in cases took the State’s total tally to 1,28,205. As many as 5,984 people have succumbed to COVID-19 in Maharashtra. Tamil Nadu is the second most affected State with 56,845 cases and 704 casualties. In the past 24 hours, the State recorded 2,396 fresh cases and 38 casualties. Inching closer to Tamil Nadu is Delhi with 56,746 cases and. The national capital reported 3,630 fresh cases and 77 casualties, taking the total tally of casualties to 2,112.
Meanwhile, the Hyderabad based Hetero Labs today received the regulatory approval from Drug Controller General of India (DGCI) to manufacture and market the antiviral drug – Remdesivir for treating COVID-19 patients. The drug will be marketed under the brand name, ‘Covifor’.
This comes a day after Glenmark Pharmaceuticals got the approval from DCGI to manufacture and market the antiviral drug, ‘Favipiravir’, with brand name ‘FabiFlu’, for treating mild to moderate cases of Coronavirus (COVID-19).
Speaking on the occasion, the Chairman of Hetero Group – B. Partha Saradhi Reddy said, “In the light of increasing Covid-19 cases in India, the approval of Covifor (Remdesivir) can prove to be a game-changer given its positive clinical outcomes. Backed by strong backward integration capabilities, we can ensure that the product is immediately made available to patients across the country.”
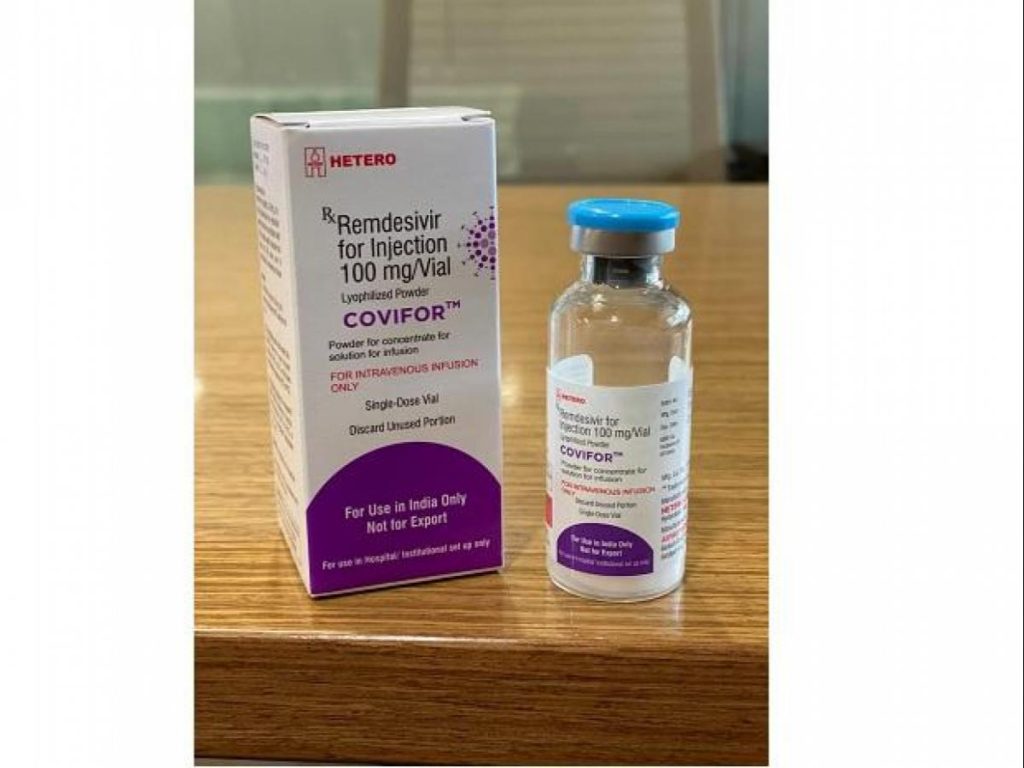
Covifor will be available in 100 mg vial (Injectable) which has to be administered intravenously in a hospital setting under the supervision of a healthcare practitioner.
The company has the Active Pharmaceutical Ingredients (API) to make 1 million doses of Remdesivir for starters. It is fully vertically integrated and would also make the Key Starting Material (KSM) for the drug.
In other developments, the Prime Minister of India – Narendra Modi today addressed the nation on the occasion of ‘International Day of Yoga’ where he highlighted how ‘Pranayam’ breathing techniques can help strengthen the respiratory system in our fight against COVID-19. With the theme of ‘Yoga at Home and Yoga with Family’, this year’s celebrations are being done digitally to ensure social distancing norms.
Addressing the countrymen, Modi said, “If we can fine tune our chords of health and hope, the day is not far away when world will witness the success of healthy and happy humanity. Yoga can definitely help us make this happen.”
In a video message, Modi also said that if we can fine tune our chords of health and hope, the day is not far away when world will witness the success of healthy and happy humanity.







